




Published on Sep 03, 2023
A hydrogen vehicle is an alternative fuel vehicle that uses hydrogen as its onboard fuel for motive power. The term may refer to a personal transportation vehicle, such as an automobile, or any other vehicle that uses hydrogen in a similar fashion, such as an aircraft. The power plants of such vehicles convert the chemical energy of hydrogen to mechanical energy either by burning hydrogen in an internal combustion engine, or by reacting hydrogen with oxygen in a fuel cell to run electric motors. The widespread use of hydrogen for fueling transportation is a key element of a proposed economy.
Hydrogen fuel does not occur naturally on Earth, and thus is not an energy source, but is an energy carrier. Currently it is most frequently made from methane or other fossil fuels. However, it can be produced from a wide range of sources (such as wind, solar, or nuclear) that are intermittent, too diffuse or too cumbersome to directly propel vehicles. Integrated wind-to-hydrogen plants, using electrolysis of water, are exploring technologies to deliver cost low enough, and quantities great enough, to compete with traditional energy sources.
Many companies are working to develop technologies that might efficiently exploit the potential of hydrogen energy for mobile users. The attraction of using hydrogen as an energy currency is that, if hydrogen is prepared without using fossil fuel inputs, vehicle propulsion would not contribute to carbon dioxide emissions. The drawbacks of hydrogen use are low energy content per unit volume, high tank age weights, the storage, transportation and filling of gaseous or liquid hydrogen in vehicles, the large investment in infrastructure that would be required to fuel vehicles and the inefficiency of production processes.
Buses, trains, PHB bicycles, canal boats, cargo bikes, golf carts, motorcycles, wheelchairs, ships, airplanes, submarines, and rockets can already run on hydrogen, in various forms. NASA uses hydrogen to launch Space Shuttles into space. There is even a working toy model car that runs on solar power, using a regenerative fuel cell to store energy in the form of hydrogen and oxygen gas. It can then convert the fuel back into water to release the solar energy. The current land speed record for a hydrogen-powered vehicle is 286.476 mph (461.038 km/h) set by Ohio State University's Buckeye Bullet 2, which achieved a "flying-mile" speed of 280.007 mph (450.628 km/h) at the Bonneville Salt Flats in August 2008. For production-style vehicles, the current record for a hydrogen-powered vehicle is 333.38 km/h (207.2 mph) set by a prototype Ford Fusion Hydrogen 999 Fuel Cell Race Car at Bonneville Salt Flats in Wend over, Utah in August 2007. It was accompanied by a large compressed oxygen tank to increase power. Honda has also created a concept called the FC Sport, which may be able to beat that record if put into production.
In a flame of pure hydrogen gas, burning in air, the hydrogen (H2) reacts with oxygen (O2) to form water (H2O) and heat. It does not produce other chemical by-products, except for a small amount of nitrogen oxides. Hence a key feature of hydrogen as a fuel is that it is relatively non-polluting (since water is not a pollutant). Pure hydrogen does not occur naturally; it takes energy to manufacture it. Once manufactured it is an energy carrier (i.e. a store for energy first generated by other means). The energy is eventually delivered as heat when the hydrogen is burned.
The heat in a hydrogen flame is a radiant emission from the newly formed water molecules. The water molecules are in an excited state of initial formation and then transition to a ground state, the transition unleashing thermal radiation. When burning in air, the temperature is roughly 2000°C. Hydrogen fuel can provide motive power for cars, boats and aero planes, portable fuel cell applications or stationary fuel cell applications, which can power an electric motor. The current leading technology for producing hydrogen in large quantities is steam reforming of methane gas (CH4). Other methods are discussed in the Hydrogen Production article. Primarily because hydrogen fuel can be environmentally friendly, there are advocates for its more widespread use.
At present, however, there is not a sufficient technical and economic infrastructure to support widespread use. The proposed creation of such an infrastructure is referred to as the hydrogen economy. At the gas pressure that hydrogen is typically stored, hydrogen requires four times more storage volume than the volume of gasoline that produces the equivalent energy, but the weight of this hydrogen is nearly one third that of the gasoline. With regard to safety from unwanted explosions, hydrogen fuel in automotive vehicles is at least as safe as gasoline. The advantages and disadvantages of hydrogen fuel compared to its competitors are discussed at hydrogen economy.
A hydrogen vehicle is an alternative fuel vehicle that uses hydrogen as its on-board fuel for motive power. The term may refer to a personal transportation vehicle, such as an automobile, or any other vehicle that uses hydrogen in a similar fashion, such as an aircraft. The power plants of such vehicles convert the chemical energy of hydrogen to mechanical energy either by burning hydrogen in an internal combustion engine, or by reacting hydrogen with oxygen in a fuel cell to run electric motors. The widespread use of hydrogen for fueling transportation is a key element of a proposed hydrogen economy.
Hydrogen fuel does not occur naturally on Earth, and thus is not an energy source, but is an energy carrier. Currently it is most frequently made from methane or other fossil fuels. However, it can be produced from a wide range of sources (such as wind, solar, or nuclear) that are intermittent, too diffuse or too cumbersome to directly propel vehicles. Integrated wind-to-hydrogen plants, using electrolysis of water, are exploring technologies to deliver cost low enough, and quantities great enough, to compete with traditional energy sources. Many companies are working to develop technologies that might efficiently exploit the potential of hydrogen energy for mobile users. The attraction of using hydrogen as an energy currency is that, if hydrogen is prepared without using fossil fuel inputs, vehicle propulsion would not contribute to carbon dioxide emissions.
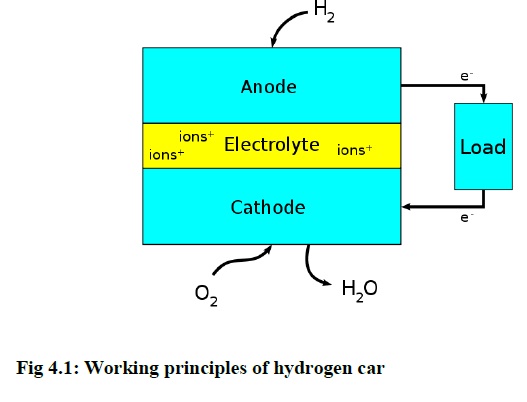
The hydrogen gas is produced by mixing the KOH and water with the help of cathode and anode terminals. This is called Fuel cell arrangement. A fuel cell is an electrochemical cell that converts a source fuel into an electrical current. It generates electricity inside a cell through reactions between a fuel and an oxidant, triggered in the presence of an electrolyte. The reactants flow into the cell, and the reaction products flow out of it, while the electrolyte remains within it. Fuel cells can operate continuously as long as the necessary reactant and oxidant flows are maintained. Fuel cells are different from conventional electrochemical cell batteries in that they consume reactant from an external source, which must be replenished – a thermodynamically open system. By contrast, batteries store electrical energy chemically and hence represent a thermodynamically closed system. Figure 4.1 shows the working principle of the hydrogen car.
After becoming familiar with the physical characteristics of hydrogen, the engine criteria can be focused on. The initial goal was to design and develop a working hydrogen fueled internal combustion engine without extraordinary modifications. This chapter will discuss basic engine theory with a description and specifications of the engine used in this research. Before an engine is selected, designed, and built some engine theory is important to know. Most engines used in automobiles are known as four stroke engines. The four-stroke cycle means that each cylinder requires four strokes of its piston, or two crankshaft revolutions. Descriptions of the four strokes are as follows.
Intake Stroke - On the intake stroke the piston moves from the top to the bottom of the cylinder. The intake valve is open creating a constant pressure increase in volume. The air/fuel mixture is pulled into the combustion chamber.
Compression Stroke - During the compression stroke, both intake and exhaust valves are closed and the piston is driven up the cylinder. As the piston moves upward the air/fuel mixture is compressed and the pressure in the cylinder increases. Toward the end of the stroke the sparks plug fires giving enough energy to ignite the air/fuel mixture.
Expansion Stroke, or Power Stroke - As a result of the air/fuel mixture ignited the piston is pushed downward by the flame front causing the crankshaft to rotate. An increase in volume is experienced.
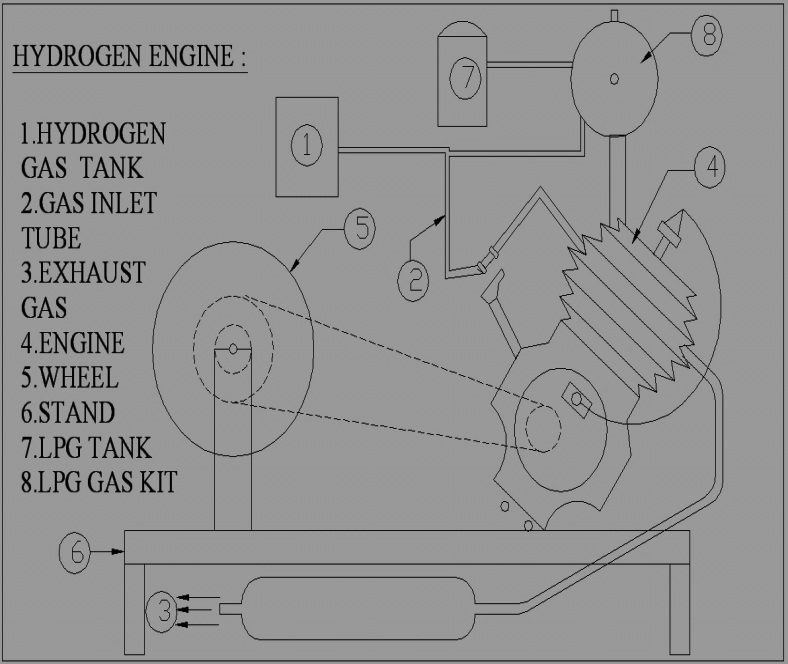
Exhaust Stroke - On the piston's way back up the cylinder, the exhaust valve is open while the intake valve is closed. The burnt gases are pushed out of the cylinder and the process is ready to begin again. Figure 4.3shows the Schematic view of Hydrogen engine (Two wheeler).
Hydrogen cars are beneficial for the environment in a number of ways. For example, they do not emit greenhouse gases that are harmful to the welfare of the ecosystem.
These cars are much more fuel efficient than gasoline vehicles, and let out less pollution overall. However, there are many drawbacks to using hydrogen-powered vehicles, though scientists are working to eliminate these downsides.
The main objective of using hydrogen cars is to save the environment from the negative impacts of burning fossil fuels. According to greenliving.com, hydrogen fuel is better because it does not release carbon dioxide into the air. Hydrogen cars also give more mileage as compared to gasoline-powered vehicles; for example, a car using hydrogen fuel can go up to twice the mileage as a gasoline car with the same amount of fuel.
Another advantage of hydrogen cars is the engine's strength and durability. Many other types of engines cannot work properly in high temperatures, and tend to overheat. Hydrogen engines, however, can work in extremely high temperatures, plus the engines do not corrode as easily as their gasoline counterparts.
There is a disadvantage around the cost of hydrogen fuel: the initial expenditure convert the infrastructure from gasoline to hydrogen is huge. It would cost billions of dollars to replace all of the current gas stations with hydrogen fueling stations.
Another disadvantage of hydrogen fuel cars is the difficulty of obtaining liquid hydrogen to use as a fuel. Hydrogen is not readily gotten from air, so it must be obtained from water molecules. There are several ways for hydrogen to be extracted from water, but none are efficient and all are very expensive.

It mainly consists of the following
1. Four stroke engine-100 cc, 6000 RPM, 7BHP
2. Sprocket with chain- Pitch 3/8, Ratio 3:1
3. Wheel Diameter -300 mm, Width 100 mm
4. Electronic unit- Anode, Cathode
5. Hydrogen tank- Capacity 2.5 litres
6. L.P.G Tank -Capacity 2 litre
7. Gas cutter
8. Operating voltage-240
9. Hose
The hydrogen infrastructure consists mainly of industrial hydrogen pipeline transport and hydrogen-equipped filling stations like those found on a hydrogen highway. Hydrogen stations which are not situated near a hydrogen pipeline can obtain supply via hydrogen tanks, compressed hydrogen tube trailers, liquid hydrogen tank trucks or dedicated onsite production.
Hydrogen use would require the alteration of industry and transport on a scale never seen before in history. For example, according to GM, 70% of the U.S. population lives near a hydrogen-generating facility but has little access to hydrogen, despite its wide availability for commercial use. The distribution of hydrogen fuel for vehicles throughout the U.S. would require new hydrogen stations costing, by some estimates, 20 billion dollars. and 4.6 billion in the EU. Other estimates place the cost as high as half trillion dollars in the United States alone.
The California Hydrogen Highway is an initiative to build a series of hydrogen refueling stations along that state. These stations are used to refuel hydrogen vehicles such as fuel cell vehicles and hydrogen combustion vehicles. As of March 2011, the California Fuel Cell Partnership showed 20 stations in operation, with eight more planned. These are located mostly in and around Los Angeles, with a few in the Bay area South Carolina also has a hydrogen freeway project, and the first two hydrogen fueling stations opened in 2009 in Aiken and Columbia, South Carolina. According to the South Carolina Hydrogen & Fuel Cell Alliance, the Columbia station has a current capacity of 120 kg a day, with future plans to develop on-site hydrogen production from electrolysis and reformation. The Aiken station has a current capacity of 80 kg. There are several funding projects for Hydrogen fuel cell research and infrastructure in South Carolina. The University of South Carolina, a founding member of the South Carolina Hydrogen & Fuel Cell Alliance, received 12.5 million dollars from the Department of Energy for its Future Fuels Program.
A significant improvement in total vehicle efficiency through an advanced hydrogen ICE design will impact on the volume of H2ICE powered vehicles in operation in a future hydrogen economy. The development of a suitable IC engine design specifically for hydrogen fuel is yet to be undertaken and the potential to achieve a viable, long term outcome is therefore unknown. The lower thermal efficiency of even the ideal hydrogen IC engine as compared to current fuel cell technologies must be weighed against the full life cycle cost from manufacture to disposal of the H2ICE.
The hydrogen internal combustion engine has a long way to go and converting current four-stroke automotive engines to operate on hydrogen will not provide us with a benchmark that gives an indication of the full potential of the hydrogen ICE. The important decision is to invest R&D dollars into a design which has an inherent capability of meeting the target requirements. The bright side is that it could be relatively inexpensive to explore and the result can be better than is currently anticipated.
The physical characteristics of hydrogen make operation of a hydrogen fueled internal combustion spark ignition engine considerably differs from conventional gasoline fueled engines. The flame speed of hydrogen, while the progression of the flame front is very similar to that of gasoline, dramatically affects the ignition timing. The ignition timing may be retarded considerably from gasoline engine timing. The exact ignition timing maps must be produced and tuned during actual engine operation. The physical characteristics of hydrogen can have a disadvantage when using hydrogen as a fuel. Equivalence ratio can have a dramatic effect on pre-ignition. Despite hydrogen's high equivalent octane rating its wide flammability limits cause many problems with premature ignition. Reducing hotspots in the combustion chamber and better control algorithms may overcome these disadvantages and enhance the performance of a hydrogen fueled engine.
[1] .Dempsey, J. "Module 9: Acts, Codes, Regulations and Guidelines." Energy Technology Training Center College of the Desert, 2001.
[2] .Greenhalgh, D.A.; Haq, M.Z.; Lockett, R.D.; Sheppard, C.G.W.; Wooley, R. "Wrinkling and Curvature of Laminar and Turbulent Premixed Flames." Combustion and Flame. 2002, pp. 131, 1-15.
[3]. Heywood, John B. Internal Combustion Engine Fundamentals. McGraw Hill,1988.
[4]. Eickhoff, H. "Analysis of Turbulent Burning Velocity." Combustion and Flame, 2002, pp. 128, 347-350.
[5]. Keller, J.; Lutz, A. Hydrogen Fueled Engines in Hybrid Vehicles. SAE, 2001.